What's the Matter?
The atomic (1) theory of matter was held as a philosophic
theory by the ancient Greeks, about 2400 years ago. They "considered
that the endless variety of substances known to man can be
explained if matter is assumed to be composed of small indivisible
and indestructible particles, or atoms." (Encyclopedia
Britannica, 1948)
The first real scientific advancement came with John Dalton
(1766-1844) who defined an atom as the smallest component
of a substance that has the physical properties of the substance.
This explained many experiments and opened the door to real
science. How small a bit of iron (or mercury, or any other
element) exhibited the physical properties of that element?
The answer was one atom. Two or a thousand or one million
atoms of mercury are still mercury and behave the same as
half as much or twice as much.
There are ninety-some elements known to occur here naturally.
They have names such as Hydrogen, Helium, Lithium, Beryllium
Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Sodium, Magnesium,
Chlorine, Nickel, Iron, Tin, Tungsten, Uranium, Calcium, Zinc
and many others. There is an arrangement called the Periodic
Table that shows the repeating patterns among these different
elements.
Elements can combine in certain proportions with each other.
Two atoms of Hydrogen can combine with one of Oxygen to make
something we call water. Combinations of atoms are called
molecules. One molecule is the smallest amount of any substance
made of a combination of atoms that has the same physical
properties as a larger collection of those molecules.
Molecules are reasonably stable combinations of atoms, but
absolutes are unachievable and so under severe stress things
decompose. A molecule of Methane (natural gas) can, if it
is hot enough (burns) combine with Oxygen in a chemical reaction
that liberates energy. Methane has one atom of carbon and
four of Hydrogen. The molecule looks like this---

into water H-O-H and Carbon Dioxide O=C=O
Notice that Carbon has four bonds (where it connects to
another atom) available, Hydrogen has one bond available and
Oxygen has two bonds available. Notice that no atoms are gained
or lost in the reaction:
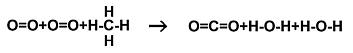
Oxygen has two bonds available but [O] is really unstable
by itself and so Oxygen gas exists in the atmosphere of this
planet as O2 or O=O . Hydrogen gas is similarly unstable by
itself and so Hydrogen gas is H-H or H2 .
The fact that these atoms combine with each other in certain
proportions---because they have 1, 2, 3, 4, 5, 6, or 7 bonds
available---was discovered by Dalton---and named by him the
Law of Definite Proportions.
1. from the Greek atomos meaning INDIVISIBLE
The atmosphere of this planet is about 21% Oxygen, 78% Nitrogen,
1/2% Carbon Dioxide and small amounts of other gasses.
It took about another hundred years for a few guys (Werner
Heisenberg, Nils Bohr, Albert Einstein, etc., etc.) to work
out WHY atoms only combined with other atoms in certain definite
proportions and what these things by which they held onto
each other, these things called bonds, actually were.
Biology The study of molecules
such as are found in living things
and how they work together
Chemistry The study of atoms and how they
combine to make molecules
Physics The study of matter and how
it behaves and of what it is made
At this point the study moved from the realm of chemistry
into the realm of physics. It turns out that atoms, while
they were thought to be indivisible, are themselves made of
components.
'Tis said that fleas
have smaller fleas
upon their backs
to bite 'em
and these in turn
have smaller fleas
and so on, ad infinitem (2).
The simplest atom is the Hydrogen atom. It has one fairly
heavy lump of mass in the middle and a little bitty thing
orbiting around it. If that atom were as big as a football
field, the lump of mass in the middle would be the size of
a housefly.
The lump of mass is not much mass---it takes about 100,000,000,000,000,000,000,000,000
of them to make a pound. The little bitty mass orbiting around
the center is much smaller---it takes 1,836 of them to add
up to one of the bigger lumps of mass in the center.
Gravitational forces are completely insignificant on this
scale. Gravity is the name given to the force that attracts
one mass to another because of their masses and the distance
between them. Gravity keeps the earth in orbit around the
sun, and the moon in orbit around the earth, and your body
stuck to the surface of this planet.
For things as small as atoms to stay together---the little
bitty thing is orbiting the great big heavy thing at hundreds
of thousands of miles per hour---so fast that physicists are
never really sure exactly where it is at any given moment---there
must be some other attractive force to balance the centrifugal
force felt by the little bitty thing orbiting around the thing
in the center.
2) Latin---means "to infinity"
There is a more powerful short-range force that exists in
this universe. It seems a very fundamental force although
we really do not know a lot about it (we physicists do, however,
have some really nifty theories). We call the characteristic
associated with the force charge. There are two kinds of charge.
We call them positive and negative because they come in equal
and opposite characteristics. The forces associated with charge
are easily measurable. Opposite kinds of charge attract each
other. Like kinds of charge repel each other. The particles
that have this quality, charge, can move around. A flow of
charged particles is called a current.
The big heavy lump in the middle of an atom is called the
nucleus of the atom. In the simplest atom, Hydrogen, the only
thing in the nucleus is this lump of mass we talked about
earlier. It has a charge which is equal and opposite to the
charge of the little bitty thing in orbit around it. Arbitrarily,
the thing in the middle got its charge called positive and
the little bitty thing got its charge called negative.
The little bitty negatively charged things could (a few of
them) be dislodged by rubbing a bit of amber (the hardened
tree sap) with some fur or silk. Sometimes there would be
a bright flash of light as a spark jumped between the pieces.
The word ELECTRIC goes back to Latin electricus, meaning produced
from amber by friction, from Middle Latin of amber, from Latin
ELECTRUM, meaning amber which was known by the name electrum,
from the Greek ELEKTRON, akin to ELEKTOR, meaning BEAMING
SUN. So, the little bitty thing with the negative charge is
called an electron. The heavy lump in the middle is called
a proton, from the Greek proton, the neuter gender version
of protos, meaning first, and Greek pro- meaning before.
All the other elements have in the nucleus not only protons
but some proton-electron pairs stuck together. Opposite charges
attract, and the electron can actually go inside the proton
and get stuck there. A nucleus may contain some of these combination
units as well as plain protons. A combination unit has mass
but no net charge (The proton and the electron have equal
and opposite charges so one of each adds up to zero.) and
since it is electrically neutral it got named a neutron.
That's pretty much what matter seems to be made of.
Copyright c 2002 Steve Smith all rights reserved
|